The effect of a 'dose' is not so simple.
"The dose makes the poison" insists that large doses always have greater effects than small doses.
But that simplistic approach overlooks greater harm being found at extremely small doses.
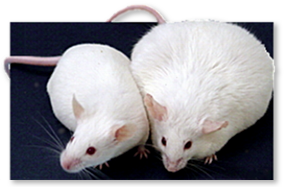
These mice are genetically identical and shared the same
diet.
The mouse on the left is normal.
The mouse on the right was exposed to 1 ppb DES while in the womb.
The mouse on the left is normal.
The mouse on the right was exposed to 1 ppb DES while in the womb.
For years it was assumed that such low exposure would have no effect.
Until someone checked.
It seems intuitive that a high dose of a toxicant will always be more harmful than a lower dose.
And if all toxics behaved exactly the same way that might hold true.
But the effect of high doses cannot always be extrapolated to predict what happens at extremely low doses.
The effect of a 'dose' is not that simple.
Factors that must be considered include
• Magnitude
• Duration
• Rate of absorption
• Timing
• Individual metabolics
• State of health and nutrition when exposed
• Concurrent exposure to other toxicants — including order of exposures and any synergies
• Duration
• Rate of absorption
• Timing
• Individual metabolics
• State of health and nutrition when exposed
• Concurrent exposure to other toxicants — including order of exposures and any synergies
When very low doses cause greater effects than higher doses the situation is called 'hormesis'.
Non-monotonic dose-response (NMDR) curves graphically describe hormesis.
In the illustration below, the black line represents a
traditional dose-response.
First, effects from a large dose are measured.
Then smaller doses are tested until no adverse effects are observed (NOAEL).
The orange line represents a dose-response discovered by testing at ultra-low doses.
The specific effect may be different but it is stronger.
First, effects from a large dose are measured.
Then smaller doses are tested until no adverse effects are observed (NOAEL).
The orange line represents a dose-response discovered by testing at ultra-low doses.
The specific effect may be different but it is stronger.
This data review looked at nearly 57,000 dose-response studies representing the effects of more than 2,100 separate drugs considered for tumor treatment.
It found that the threshold model ('dose makes the poison') couldn't explain the data.
Instead, hormesis was found 4x more often than chance would predict.
The hormetic model did a superior job of interpreting low-dose toxic responses.
Exactly how can a chemical exhibit one kind of effect at very small levels and a different effect — or no effect at all — at higher levels?
That is a reasonable question, and one that new research seeks to answer more fully.
One explanation is that
• At low levels a chemical may change the ratio of
receptors stimulated
• At higher levels the receptors are completely overwhelmed.
• At higher levels the receptors are completely overwhelmed.
Another explanation is that
• At low concentrations a chemical may influence cell
behavior without exhibiting damage
• At higher concentrations the cells are actually damaged or destroyed
• At higher concentrations the cells are actually damaged or destroyed
In some circumstances both explanations may be correct.
Hormones play specific roles, at specific moments in time, throughout a person's life.
Hormones help to regulate biological processes by binding to cell receptors and signaling the processes to start, stop, speed up, or slow down.
Sort of like a dimmer switch on a light.
Some chemicals resemble hormones.
These chemicals are known as endocrine disruptors.
They can mimic hormones, block them, or have other effects.
If the actions of hormones are prevented, interrupted, or increased then the effects can range from subtle to dramatic.
For example, exposure occurring at a young age can cause
a subtle change in how a gene expresses itself.
This sets up a hidden, long-term progression of conditions that eventually lead to some form of cancer.
In other cases the original disruption might occur at a key moment during development in the womb.
The dramatic result might be a birth defect, mental retardation or miscarriage.
This sets up a hidden, long-term progression of conditions that eventually lead to some form of cancer.
In other cases the original disruption might occur at a key moment during development in the womb.
The dramatic result might be a birth defect, mental retardation or miscarriage.
The amount of chemical necessary to cause these disruptions does not have to be large.
A vanishingly small amount is all it takes — "just enough" to alter an event.
"...even infinitesimally low levels of exposure —
indeed, any level of exposure at all — may cause
endocrine or reproductive abnormalities, particularly
if exposure occurs during a critical developmental
window.
Surprisingly, low doses may even exert more potent effects than higher doses."
Surprisingly, low doses may even exert more potent effects than higher doses."
Arsenic is a classic example of 'dose makes the poison' toxicology.
Give someone enough and they become poisoned. Below that, no problem.
But research shows arsenic disrupts many different hormone receptors at levels of just a few parts per billion — levels found in drinking water.
Chronic (low level) intake of arsenic has been associated with increased risk of cancer, diabetes, developmental and reproductive problems, and cardiovascular disease.
Recognizing arsenic's hormetic pattern helps make sense of that research — all of those illnesses can be triggered or exacerbated by hormone disruption.
Here is a link to ~30 studies describing other accounts of toxic hormesis.
If a chemical is regulated, its safety threshold was probably based on finding a level of exposure that causes no harm — the 'no observable adverse effects level' (NOAEL).
But a NOAEL is derived by starting with a high dose and then reducing subsequent doses until no affect is observed.
That approach misses other harm that can take place at even lower doses.
The emergence of hormesis poses a large problem for how agencies like the EPA and FDA do their job.
It also increases the level of risk companies must manage in the production and utilization of chemicals.
Toxics that don't have a NOAEL can still exhibit nonlinear effects at low doses.
Lead (Pb) is an example.
It is well documented that there is no safe level of Pb exposure, but it is generally presumed that the harm diminishes as exposure decreases.
This research finds that Pb-associated decrements in IQ are proportionately greater at a blood lead level below 10 μg/dL than above.
So even though harm continues to climb at higher levels of exposure, the effect is most pronounced at levels below the CDC definition of "elevated lead".
The science of hormesis is relatively new.
One place to learn more is Our Stolen Future.
This PubMed search provides a current survey of articles related to hormesis, NMDR, and toxicants.
There is still more investigation to be done, but it looks like chemicals that are endocrine disruptors at one level are often excitotoxic at another level.
Some people are under the misconception that hormetic effects are always beneficial.
In rare instances, the ultra-low dose effect of a toxicant may exert beneficial influence in narrowly defined situations (possibly, for example, radiation hormesis).
But so far, the bulk of hormetic rresearch reveals new points of harm.
Hormesis is not a case where the toxicant exerts one
effect at high doses (harmful) and the opposite effect at
low doses (beneficial).
Hormesis is a case where the toxicant exerts particular effects at higher doses (harmful) and different effects at lower doses (also harmful).
Hormesis is a case where the toxicant exerts particular effects at higher doses (harmful) and different effects at lower doses (also harmful).
Beware of attempts to manipulate hormetic data so that toxic regulations become relaxed...
Hormesis does not suggest that "a little poison is good
for you".
Hormesis shows how a toxicant is harmful across a greater range of doses.
Hormesis shows how a toxicant is harmful across a greater range of doses.
Investigating toxicants at low levels is important research, and hormetic modeling is a valuable contribution towards improving global health and safety.
Unfortunately there are tens of thousands of chemicals suspected to be toxic — but for which no studies have been done of any kind.
No idea how they affect the brain, or developing fetuses, or how they interact with other toxics.
Nothing.
But they are showing up in our bodies.
EWG's "Ten Americans"
presentation